バイオ医薬品
製造
Controlled, aseptic environments
The preparation of media and dosing solutions for upstream processes, along with buffers for downstream applications, is critical to achieving reproducible and efficient biopharmaceutical production. Media typically contain carbohydrates (e.g., glucose), nitrogen sources (e.g., amino acids), lipids, and trace salts - all essential for supporting protein synthesis.
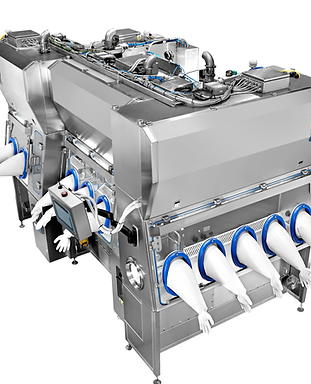
These components are usually supplied in powder form and must be dissolved in high‑purity Water for Injection (WFI). To meet strict cleanliness standards, powders are introduced into preparation tanks under controlled, aseptic conditions in compliance with cleanroom protocols. Once prepared, the solutions are transferred to storage tanks and distributed to reactors or vessels as needed.
Depending on the process stage, these solutions serve as culture media, correction media, fed‑batch media, or buffers for downstream processing.


The PTS Powder Transfer System® offers an optimal solution for charging powders into media vessels under controlled and aseptic conditions. This innovative system can be sterilized alongside the vessels, ensuring that the entire process adheres to the highest standards of hygiene and safety.